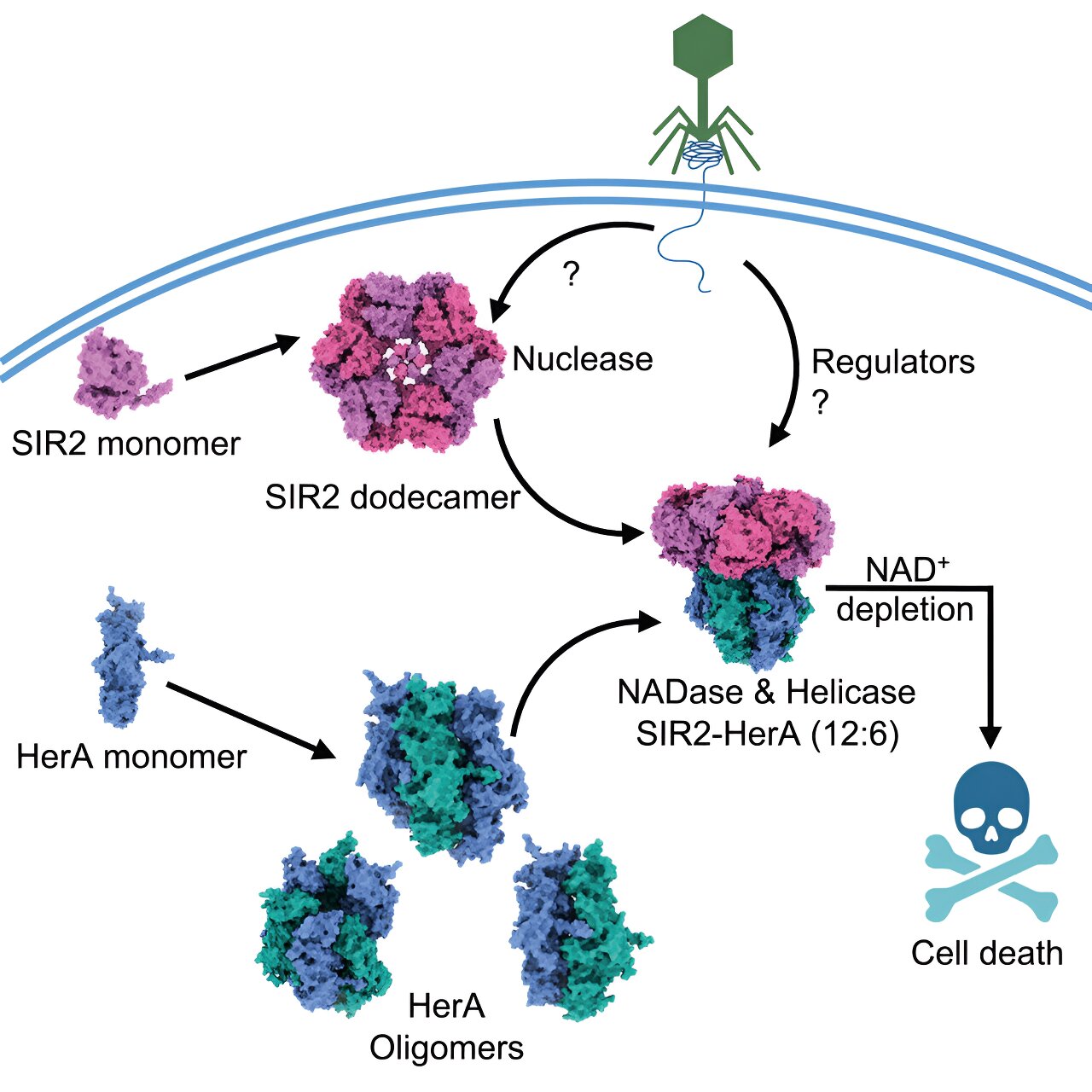
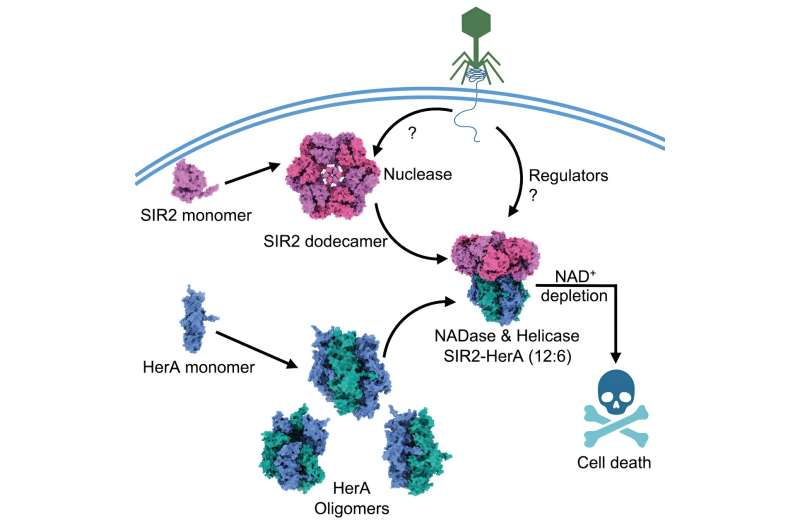
Scientists have revealed a never-before-seen phenomenon in a protein: Alone, the enzyme processes DNA and RNA but, when bound to another protein as part of a defense system, interacts with a completely different type of compound to help bacteria commit suicide.
The finding came about as the researchers focused on detailing how this defense mechanism works in bacteria that are infected by phages, viruses that invade and make copies of themselves inside bacterial cells. In addition to detailing the proteins’ structures and binding sites, the experiments unearthed this unprecedented switch in enzymatic functions.
“This was a big discovery,” said senior study author Tianmin Fu, assistant professor of biological chemistry and pharmacology in The Ohio State University College of Medicine. “When proteins form a complex, that usually increases or decreases an enzyme’s activity—but we’ve never seen a complete switch in function. That’s entirely new to the enzymology field.”
In the bigger picture, he said, a better understanding of how bacteria use defense systems to die versus staying infected by phages could be translated into therapies that convince cancer cells to program their own death as well.
“If we could introduce this type of system into a cancer cell, that could lead to the development of a new strategy for cancer treatment,” said Fu, also an investigator at the Ohio State University Comprehensive Cancer Center.
The research is published in Molecular Cell.
When infected by phages, bacteria opt for death to prevent phages from taking over a bacterial community. The complex examined in this study, the combination of proteins called SIR2 and HerA, was identified along with hundreds of other bacterial defense systems in previous research that focused on genomic analyses.
In an E. coli model, Fu and colleagues used cryo-electron microscopy to determine the biochemical structures of the proteins alone and during and after their assembly as a supramolecular complex.
“This system has been identified in many different bacteria, and though we studied it in E. coli, we think it would function very similarly in other bacteria,” Fu said.
The analysis suggested that SIR2 and HerA have an affinity for each other, showing that SIR2’s wheel-like structure functions as an organizer of HerA molecular clusters before the two settle into a complex consisting of six identical molecular subunits. However, exactly what triggers their connection is still a mystery.
Results showed that once assembled, the complex could exist in bacteria without incident, suggesting bacteria somehow inhibit the system’s defense activity unless a phage enters the scene. When phages were introduced, the bacteria quickly died—by their own design because the defense system had been activated to deplete a small molecule called NAD+ that bacteria require to survive. That activation mechanism remains unknown for now, as well.
Experiments confirmed that SIR2 was responsible for discarding the NAD+, which was a surprise. SIR2’s first job as a nuclease is digesting nucleic acids to maintain proper cell functions. But when bound to HerA and activated as part of the defense system, its enzymatic function switched—SIR2 became an entirely different type of enzyme called NADase, which generates a water-based reaction to dissipate NAD+.
“We now want to address this huge, fundamental biological question—how does complex assembly switch SIR2’s activity from a nuclease to a NADase?” Fu said. “Figuring out this mechanism would be big for the field, and this system is extremely interesting because it has so many different enzymatic activities in one preassembled complex.”
Fu also envisions a synthetic biology toolbox of the future in which bacterial tricks are adapted into cancer cell-killing strategies. “We’re starting to learn from bacteria, and hopefully we can reprogram them into powerful tools for cancer diagnosis and treatment,” he said.
More information:
Zhangfei Shen et al, Assembly-mediated activation of the SIR2-HerA supramolecular complex for anti-phage defense, Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.11.007
Provided by
The Ohio State University
Citation:
Research reveals a rare enzyme role change with bacterial defense system assembly (2023, December 13)
retrieved 13 December 2023
from https://phys.org/news/2023-12-reveals-rare-enzyme-role-bacterial.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.





