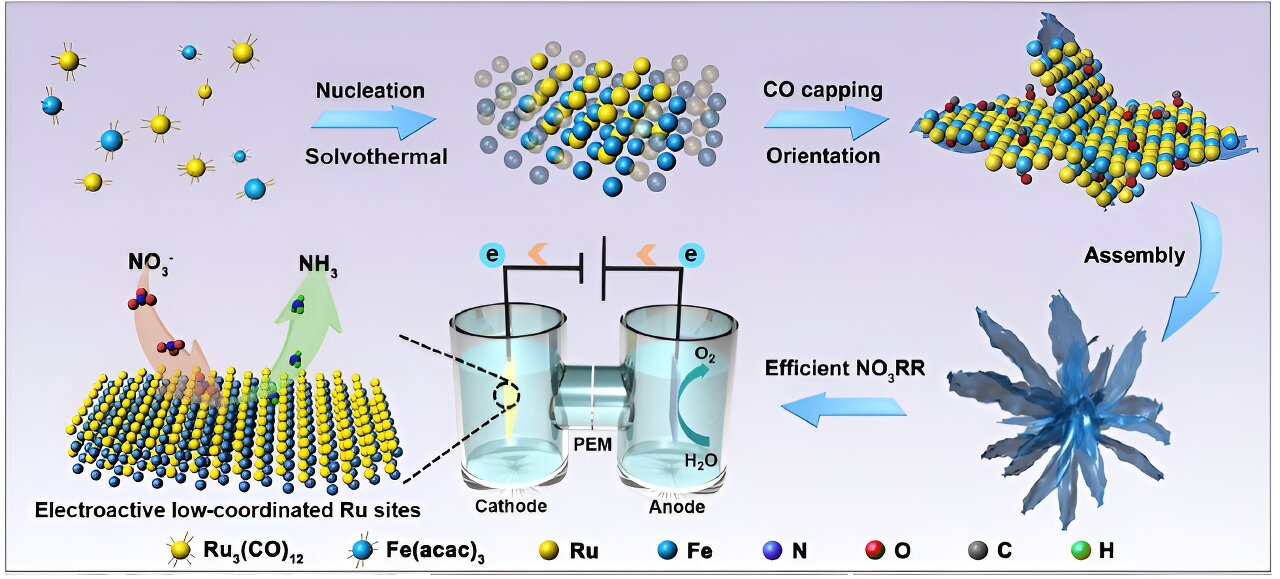
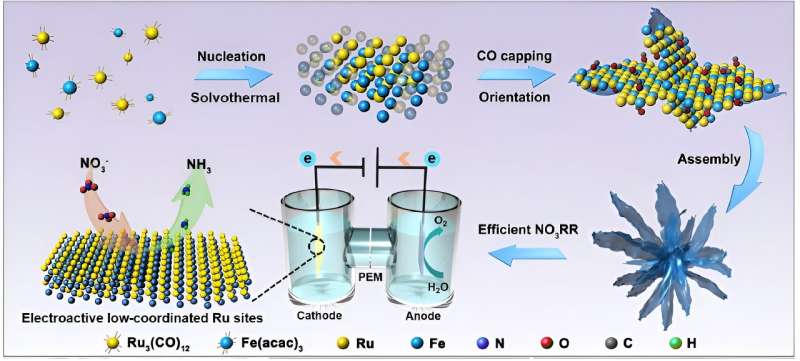
Ammonia (NH3) is regarded as a promising carbon-free energy carrier, but its energy-intensive production process still challenges global scientists. A research team led by City University of Hong Kong (CityU) recently engineered a bimetallic alloy as an ultrathin nanocatalyst that can deliver greatly improved electrochemical performance for generating ammonia from nitrate (NO3–), offering great potential for obtaining carbon-neutral fuel in the future.
The findings were published in the journal the Proceedings of the National Academy of Sciences (PNAS) under the title “Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate.”
Ammonia, which is commonly used in fertilizer, has recently attracted a lot of attention because it can provide a source of hydrogen for fuel cells, and it is easier to liquefy and transport than hydrogen. Owing to its huge demand, upcycling nitrate (NO3–) from ammonium fertilizer-polluted wastewater has emerged as an alternative for reproducing valuable ammonia and making agriculture more sustainable.
Currently, electrochemical nitrate reduction reaction (NO3RR) is regarded as a promising solution for ammonia synthesis. It comprises mainly deoxygenation and hydrogenation steps (i.e. NO3– + 9H+ + 8e– ➙ NH3 + 3H2O) with metal-based electrocatalysts.
“However, the undesired by-products and the competing hydrogen evolution reaction (HER) during NO3RR apparently hinders the yield rate of ammonia production,” said Professor Fan Zhanxi, of the Department of Chemistry at CityU, who led the study.
Instead of modulating the electrocatalysts’ size or dimension, as other previous research did, Professor Fan’s team focused on improving the active sites, where substrate molecules bind and catalysis takes place on the surface of the electrocatalysts.
“Ruthenium (Ru) is an emerging material as an electrocatalyst for NO3RR, but it also has the problem of favoring HER, which leads to its active sites being highly occupied by undesired active hydrogen, leaving insufficient area for nitrate reduction into ammonia,” explained Professor Fan.
To overcome the challenges, the team introduced another metal—iron (Fe)—to modulate the atomic coordination environment of the active sites. By changing the coordination environment of the Ru sites, the electronic structures and surface properties of Ru and hence their catalytic activity for producing ammonia are optimized. To further enhance the electrocatalyst performance, the team developed a one-pot synthesis approach for making ultrathin nanosheets that are assembled as a flowerlike structure—called RuFe nanoflowers.
This novel bimetallic alloy made-electrocatalyst possesses a highly stable electronic structure due to the complementary orbitals that reach efficient electron transfer and robust valence states, which also suppresses the competitive HER and lowers the energy barriers for NO3RR. Moreover, the electrochemically active surface sites of the RuFe nanoflowers measured 267.5 cm2, much larger than the 105 cm2 for Ru-nanosheets for the reactions to take place.
Remarkably, RuFe nanoflowers demonstrated much better electrochemical performance, with an outstanding charge transfer efficiency, known as faradaic efficiency (FE), of 92.9% and a yield rate of 38.68 mg h−1 mgcat−1 at −0.30 and −0.65 V for ammonia production, which is almost 6.9 times that of sole Ru-nanosheets.
“This research indicates great potential for RuFe nanoflowers in next-generation electrochemical energy systems,” said Professor Fan. “We believe this work can stimulate follow-up studies on modulating the atomic coordination environment of active sites in metal-based catalysts for ammonia production, further promoting a sustainable nitrogen cycle to achieve carbon-free energy in the future.”
More information:
Yunhao Wang et al, Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2306461120
Provided by
City University of Hong Kong
Citation:
Bimetallic alloy nanocatalyst boosts efficient ammonia production with potential for carbon-free energy (2023, December 8)
retrieved 8 December 2023
from https://phys.org/news/2023-12-bimetallic-alloy-nanocatalyst-boosts-efficient.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.





