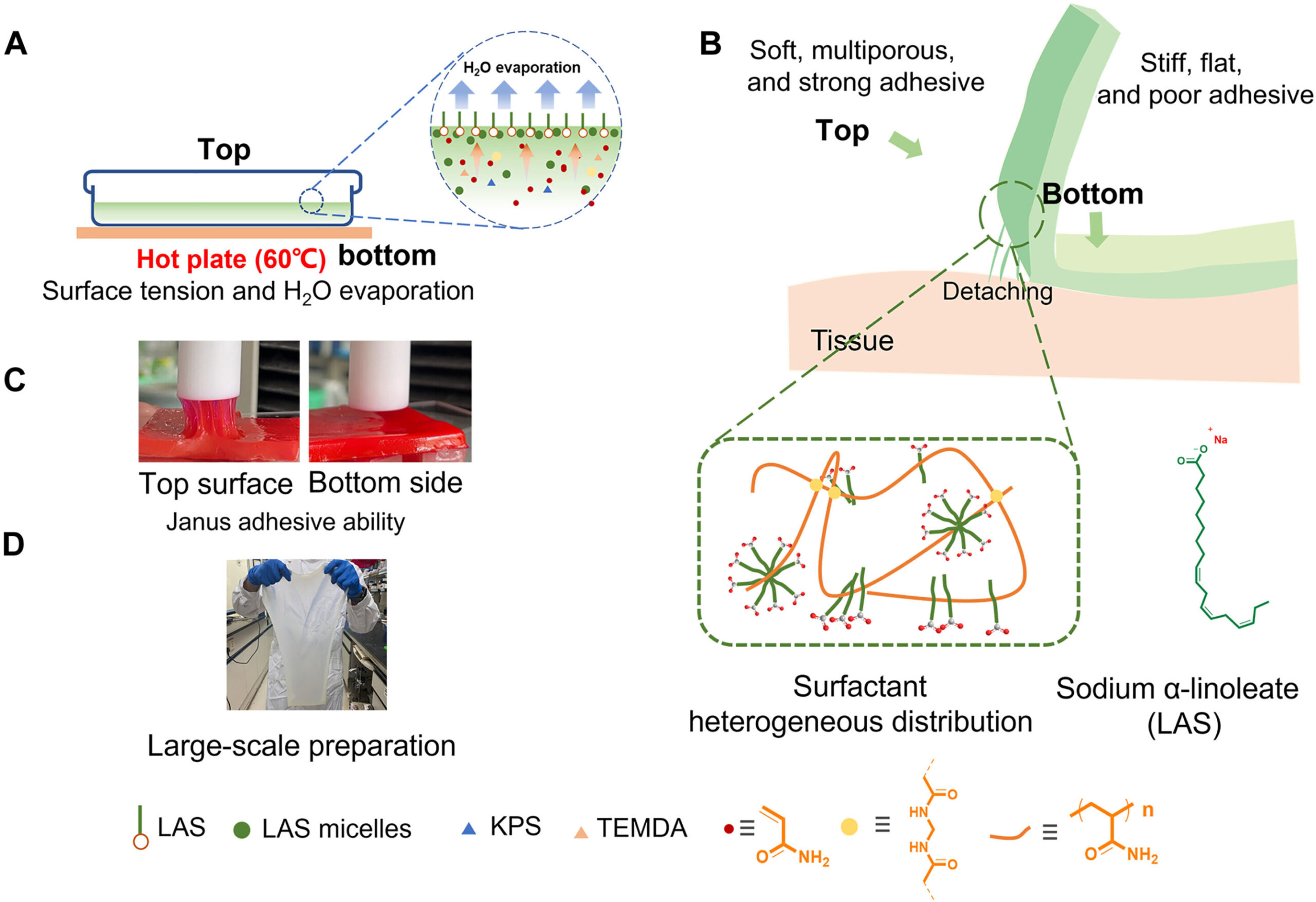
![Janus adhesive hydrogel synthetic process exhibition and characterization. The schematic diagram shows an open-face reaction container, consisting of a box with a lid and illustrates the approach for producing the Janus adhesive hydrogel involving the aggregation of the LAS component, which is driven by surface tension and an evaporation effect (A). The schematic illustrates the contrasting properties between the top and bottom surfaces of the Janus hydrogel (B). The digital photography (C) demonstrates adhesive difference between the top/bottom sides [hydrogel stained with Rhodamine 6G, substrate: PTFE (polytetrafluoroethylene)]. The digital photography (D) demonstrates the feasibility of large-scale synthesis of Janus hydrogel using a commercial cake pan (280-mm by 230-mm by 3-mm size of hydrogel is presented). KPS, potassium persulfate; TEMDA, N,N,N′,N′-tetramethylethylenediamine. Credit: Science Advances, doi: 10.1126/sciadv.adj3186 One-step synthesis of Janus hydrogel](https://scx1.b-cdn.net/csz/news/800a/2023/one-step-synthesis-of.jpg)
Janus adhesive hydrogels hold promising applications across health care fields. Nevertheless, a simple method to synthesize the material had yet to be bioengineered in the lab.
In a new study now published in Science Advances, Huowen Chen and a research team in China devised a simple method to prepare Janus hydrogels based on fundamental phenomena including the self-aggregation of surfactants at high concentrations at the air/water interface.
The team combined a small amount of sodium-alpha-linoleate with acrylamide through free radical polymerization and synthesized the Janus adhesive hydrogels. These constructs showed remarkable adhesive strength, chemical properties, and surface morphology, which the team investigated using molecular dynamics simulations to understand the mechanisms of the biomaterial’s properties.
Hydrogels—the basics
Three-dimensional network materials of hydrogels are primarily made of hydrophilic polymer chains that contain a large amount of water. Due to their excellent biocompatibility, wetting properties and flexibility, the materials are well-suited across biomedicine.
To accomplish this, hydrogels must have good adhesive properties with biological tissue. Bioengineers have recently designed adhesive hydrogels by introducing host-guest supramolecular, amino acid, and nucleic acid groups. Janus hydrogels can be created with chemical or physical differences between the top and bottom sides of the hydrogel, through soaking methods, stepwise synthesis, or mold casting.
As a result, it is crucial to develop a simple, efficient, and cost-effective environmentally friendly preparation method to apply the Janus adhesive hydrogels. In this work, Chen and colleagues proposed a new study to synthesize Janus adhesive hydrogels by exploring their interfacial adhesion.
During the experiments, they used a plant-derived unsaturated fatty-acid derivative combined with commercial monomer acrylamide, micelles, and sodium-alpha-linoleate as the base components. The proposed method is a first-in-study plan to overcome the challenges of Janus hydrogel production and scale-up adhesive hydrogel production.
Preparing the Janus hydrogels
The research team designed the Janus adhesive hydrogels by regulating diverse properties between each surface of the material, including its morphology, chemical composition, and hydrophilicity.
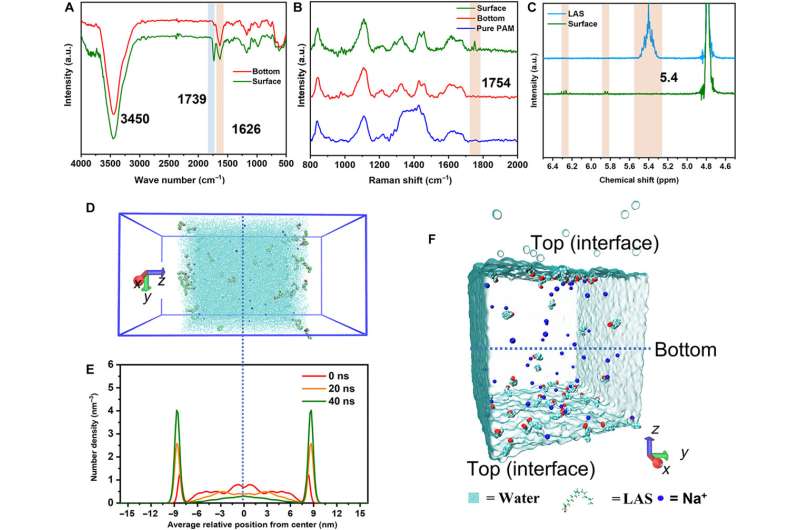
At first, they dissolved the surfactant monomer in deionized water to create a transparent homogenous solution with a concentration higher than the critical micelle concentration. Thereafter, the team added a monomer acrylamide and methylene bis-acrylamide to prepare the hydrogel and conducted a series of chemical characterizations on the surface and bottom of the hydrogel using Fourier transform infrared spectroscopy.
The scientists noted strong characteristic absorption peaks relative to the ammonia functional group and carbonyl functional group. They verified these results with Raman spectroscopy to indicate free radical polymerization of the monomer during the formation of the hydrogel.
Additional experiments
The scientists conducted molecular dynamics studies to identify the distribution of biomaterial constructs and show the aggregated molecules at the air-water interface under surface tension and thermal driving forces. The results showed how the chemical composition of the material varied across surfaces to contribute to its morphology and hydrophilicity.
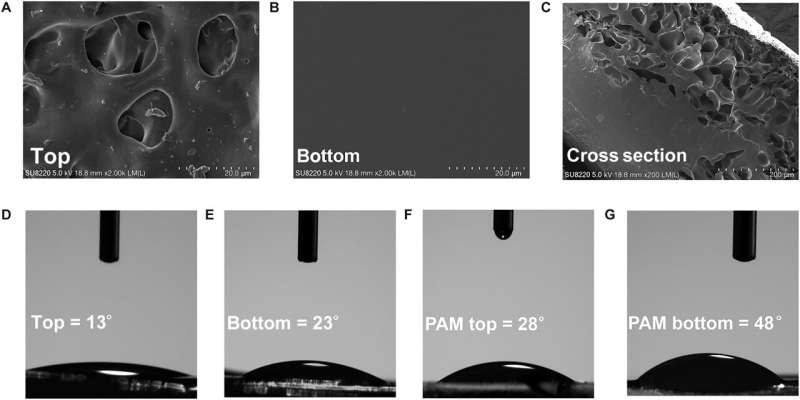
The team conducted transmission electron microscopy to reveal the distinct chemical composition and physical morphology of the material, with distinct influence on their adhesion properties to investigate mechanical and adhesive properties of the hydrogel.
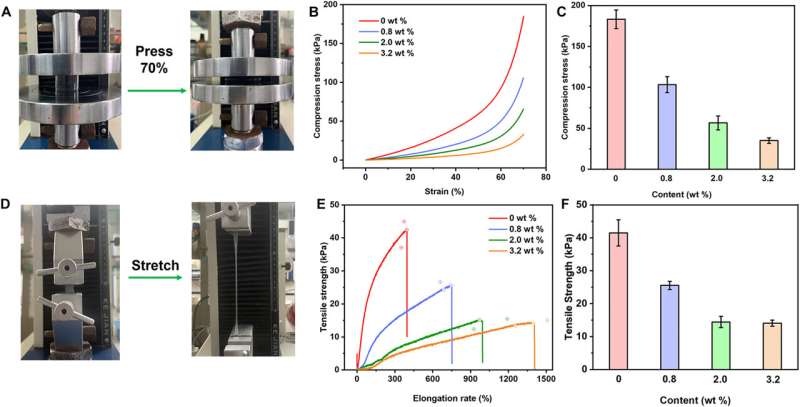
Since the bioengineered construct is a pressure-sensitive adhesive hydrogel, the scientists achieved sufficient adhesive properties by facilitating good contact with the material substrate. As the content of sodium-alpha-linoleate increased, the compressive modules of the hydrogel signaling decreased, to facilitate a compressive modulus of the hydrogel.
While low compression of the material ensured its adhesion, it required good tensile properties to adjust the physical properties of the material and achieve optimal performance. The total experimental outcomes confirmed the hypothesis that the sodium-alpha-linoleate surfactant formed diverse distributions in the hydrogel through surface tension and evaporation effects to create an adhesive Janus hydrogel material. The constituents of the construct offered strong adhesive properties and long-lasting performance.
Characterizing the biocompatibility of the material
Chen and colleagues characterized the hydrogels for a range of medical applications and ensured their biocompatibility by carefully considering two major harmless components according to previous studies.
The team validated the outcomes by performing biocompatibility tests with human skin fibroblast cells using a transwell assay and via direct contact with hydrogel materials. After further studies, the hydrogels did not exhibit toxicity towards the skin fibroblast cells to highlight excellent biocompatibility with great potential for a variety of biomedical applications including drug delivery, and skin repair.
Outlook
In this way, Huowen Chen and the research team validate the composition of Janus adhesive hydrogels by harnessing the heterogenous distribution of surfactants inspired by their natural aggregation at the water-air interface. The team accomplished one-step synthesis of the Janus adhesive hydrogel via free radical polymerization. The outcomes of the newly developed material exhibited remarkable differences in chemical composition, morphology, and mechanical properties.
![The adhesive ability and cell viability of hydrogel PAM-co-LAS. Graphics show the 90° peeling test experiment and the adhesive energy of PAM-co-LAS hydrogel with different contents of LAS component; t and b are abbreviations for top side (t) and bottom side (b), respectively (A to C). (D) Repeatable adhesive ability of PAM-co-LAS-2.0 hydrogel. (E) Long-term adhesive strength change of PAM-co-LAS hydrogel [(D and E) detected by flat punch indentation method with PTFE]. (F) Investigating the hydrogel's compatibility with human skin fibroblasts cell by a transwell assay that directly incubates the cells with hydrogel, in which the hydrogel was in direct contact with the human dermal cells. Credit: Science Advances, doi: 10.1126/sciadv.adj3186 One-step synthesis of Janus hydrogel](https://scx1.b-cdn.net/csz/news/800a/2023/one-step-synthesis-of-4.jpg)
While the top surface of the Janus hydrogel showed a relatively rough texture, the bottom side of the material showed lower adhesion energy. The distinct difference between the adhesion properties ensured the preparation of the biomaterials with the heterogeneous distribution of surfactants to provide a new, synthetic strategy to prepare Janus hydrogels for a broad range of practical applications. The ongoing outcomes offer the capacity to explore different surfactants in Janus hydrogel synthesis to investigate their biomedical potential.
More information:
Huowen Chen et al, One-step synthesis of Janus hydrogel via heterogeneous distribution of sodium α-linoleate driven by surfactant self-aggregation, Science Advances (2023). DOI: 10.1126/sciadv.adj3186
© 2023 Science X Network
Citation:
One-step synthesis of Janus hydrogel (2023, December 4)
retrieved 4 December 2023
from https://phys.org/news/2023-12-one-step-synthesis-janus-hydrogel.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.





